In July 2022, the FDA announced a proposed rule, Revising the National Drug Code Format and Drug Label Barcode Requirements.
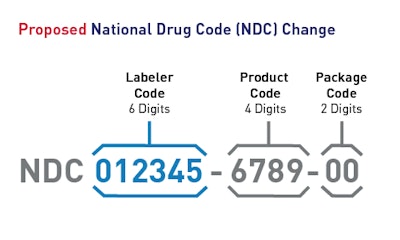
National drug codes (NDCs) are standards for identifying drugs marketed in the U.S, composed of a labeler code, product code, and package code. FDA is proposing to change the NDC to 12 digits in length with three distinct and consistent segments and one uniform format.
 Current NDC segments and formats. Source: FDA
Current NDC segments and formats. Source: FDA
As the Agency explains, the NDC proposal is intended to minimize the impact of “running out of ten-digit national drug codes (NDCs) by adopting a single, uniform 12-digit format for FDA-assigned NDCs.” FDA has approximately 10 to 15 years of available 5-digit labeler codes if they maintain an assignment rate of 1400 to 1800 labeler codes per year.
Additionally, FDA is proposing to revise the drug product barcode label requirements to accommodate advances in technology by allowing the use of unspecific automatic identification and data capture formats other than linear or nonlinear barcodes in the future without the need to revise the regulation again.
FDA is seeking perspectives on the impact that the proposed NDC change will have on industry, because it's a significant change that impacts numerous types of stakeholders including but not limited to:
- Human and animal drug manufacturer and distributors
- Insurers/payor
- Drug databanks
- Pharmacies
- Electronic health record vendors
- Hospitals, nursing care facilities, and other clinical sites
- Federal and state/local agencies
 Proposed NDC segments and formats. Source: FDA
Proposed NDC segments and formats. Source: FDA
At the HDA Traceability Seminar in October, Connie Jung, RPh, Senior Advisor for Policy, FDA, explained the rationale and urged attendees to submit comments via the Federal Register. The change is intended to provide certainty and predictability, facilitate adoption of a single NDC format by all stakeholders, and eliminate the need for stakeholders to maintain multiple versions of an NDC.
Timeline
5 years: In terms of the proposed effective date for the change, Jung noted that FDA is likely to begin assigning only six-digit labeler codes (and stakeholders would need to have systems capable of handling the new uniform 12-digit NDC) five years after the regulation is finalized.
3 years: This would be followed by a transition period of three years in which NDCs on carton labels should change to the new 12-digit format, while products labeled with 10-digit codes could remain in interstate commerce. By the end of the three-year transition period (meaning 8 years post-regulation change), all firms would be required to use a 12-digit NDC.
View and submit comments via the Federal Register here:
Comments are accepted till 11/22/2022
View the FDA release here.