The numbers are daunting. Food allergies affect 7 million to 8 million U.S. consumers. When you consider that an allergic response may range from mild symptoms to death, the thought of running different allergens on the same line can become paralyzing. In most food products, it is unrealistic and unsustainable to use harmonized formulas or to expand and isolate processing lines to keep allergens separated.
Manufacturers have used precautionary labeling as an added layer of risk communication to consumers. This may be helpful to bridge a gap when equipment has not been validated for the cleaning method, but FDA is very clear that precautionary labeling cannot be used as a substitute for good manufacturing practices that include cleaning to remove allergens from shared equipment.
Here is an example of a labeling statement from FDA: “While not due to a cross-contamination from the production line, a recent voluntary recall of ice cream for undeclared allergens highlights the risks without reward of precautionary statements. In this case, tree nuts that might include almonds, Brazil nuts and hazelnuts were not declared on the label. The recalled ice cream did include statements of ‘contains walnuts’ and ‘may contain other tree nuts,’ but the precautionary labeling when the allergen was known to be in the product, was not enough support for the manufacturer to leave the product in market.”
Perhaps as a result of the voluntary nature or the lack of clear rules for the language, precautionary labeling is often misunderstood or completely ignored by allergic consumers, sometimes causing tragic results. In recent years, the demand for clean labels has led to increased focus on sanitary design, cleaning practices and validation of cleaning procedures.
To provide different products for diverse customers and still remain competitive, manufacturers must have confidence in their food labeling and formulations. This starts with validated sanitation actions. Clearly identifying any products that contain allergenic proteins is the right thing to do for your consumer and your business.
In response to the need for a common resource for evaluating and validating allergen cleaning processes, PMMI’s OpX Leadership Network, in collaboration with the Grocery Manufacturers Association (GMA) Sanitation Share Group and the GMA Food Allergens Committee, assembled a diverse group of professionals to create Checklist: Allergen Cleaning Validation in 2017. This checklist can be used to validate a new line, an equipment change or new products brought to an existing line. Download the checklist at http://bit.ly/2JkOOgv.
Protecting consumers
Validation of sanitation programs is not currently required by the Food Safety Modernization Act, but recalls due to undeclared allergens continue to be frequent.
While undeclared allergens are most commonly reported as mixed foods or supplier errors for ingredients, failing to remove the allergen from the production line and allowing it to enter another product where the ingredient is not declared is a risk to your consumer and your business.
Validating the cleaning method applied to the equipment and ensuring no allergens are released into the production of the next food are essential. These protocols can help to provide clean labels and high confidence that the consumer is protected from undeclared allergens.
To date, I have most frequently used the OpX checklist to create formalized allergen control plans with my plant teams. The checklist reminds us of the details required and provides a framework to collect and house information across multiple sites. Also, we are beginning to navigate the validation process for allergen sanitation.
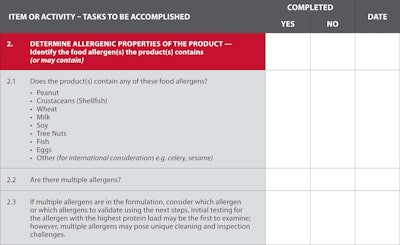
The checklist
The OpX team developed seven key areas of activity to consider when validating a sanitation process for the removal of allergens.
1. Current state, goals and known gaps.
2. Identify allergenic risks to the target food, target line or other scope of the work.
3. Evaluate and understand the equipment affected – dry vs. wet cleaning; other constraints.
4. Design of the study.
5. Conducting the study.
6. Documenting the results.
7. Reassessment rules.
Following the checklist is a concurrent set of events for my plant teams. Because the operation has been manufacturing for decades, there are established practices and knowledgeable staff to answer questions about the equipment design and the cleaning procedures (section 3 of checklist). This team also performs assessment of past and present allergen risks and any scope constraints (section 2 of checklist). We use the verification records (visual inspection, ATP swabs, protein swabs) and complaint data, if applicable, as well as industry trends and news to identify gaps and strengths of the current system (section 1 of checklist). Most commonly, the gap identified is the need to transition verbally communicated information from experienced staff into a written format, so methods are standardized, and new personnel are able to meet the same level of success when cleaning the equipment. This gap is actively closed with sanitation standard operating procedures and work instructions before we are able to move into the remaining sections of the checklist and complete validation of allergen cleaning on our lines.
Validating cleaning practices for the current equipment and processes
Once we have evaluated the risks on the line and in the facility, we establish a working model for testing our cleaning processes. We test for allergens on our lines to collect data for our reports. Allergen-specific tests and food tests are used with cleaning procedures vetted by experts in the chemistry and the removal of soils for our products. Our equipment ranges in age and contains a variety of materials, so we scrutinize both dry and wet cleaning requirements. The selection of the allergen tests is based on the number of allergens and the format of the ingredient (particulate, paste, other). If no previous evaluations with allergen tests have been completed, all the allergens are evaluated at the first validation of the cleaning. Our list is longer than the checklist because we include allergens for different countries if the line produced foods for export.
As we progress in the data collection and can demonstrate effective results, this wide variety of allergens is reduced to a core few that are the most difficult to remove. We test both food contact surfaces and food products. Sampling locations on the line begin with a wider quantity, and, once difficult-to-clean locations are identified, the sampling locations are reduced.
The final result of this process is to establish key sampling sites, food tests and targeted allergens for each line. Verification with protein or ATP swabs and visual inspection occurs after every clean, but validation of the sanitation effectiveness is less frequent and is used to confirm the sanitation practices have not drifted.
Validating (or revalidating) production lines for new products
When new products are brought to the facility, the team evaluates the allergenic risks and whether the current program for the line remains acceptable. New allergens or new forms of an ingredient containing an allergen triggers a validation (or a revalidation) of the sanitation process. If the same methods can remove the new product or allergen, then no changes are made to current processes. Less-than-acceptable results may indicate a need for changes in method, tools, time or chemicals, and a more involved assessment is initiated. Essentially, the checklist starts over again.
Using a science-based, systematic process to validate the cleaning methods used to remove allergens is more than what is expected by current regulatory rules, but is the most defensible position in preparation for any future scrutiny.