Key Takeaways:
- FDA clears Medtronic’s Altaviva, an implantable tibial neuromodulation device for urge urinary incontinence.
- Adoption will hinge on reimbursement and evidence: detailed TITAN 2 outcomes and label specifics haven’t been published yet, so payers and clinicians will look for peer‑reviewed data on efficacy, durability, reinterventions, and safety.
- Medtronic positions Altaviva next to InterStim and asserts it now offers the only full neuromodulation portfolio for bladder control (the company’s characterization).
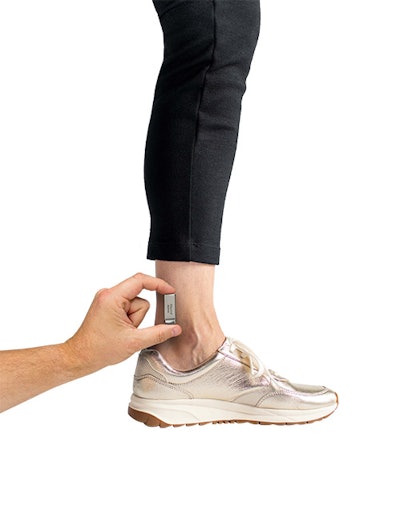
Medtronic has received U.S. Food and Drug Administration approval for Altaviva, an implantable tibial neuromodulation (ITNM) device designed to treat urge urinary incontinence, expanding the company’s pelvic health portfolio and offering physicians a minimally invasive alternative to drug therapy and sacral neuromodulation.
The device is implanted near the ankle in a procedure that does not require sedation or imaging, according to the company. Patients leave the procedure with therapy activated. Altaviva delivers electrical impulses to the tibial nerve to help regulate bladder control. Medtronic says the system is MRI compatible, has an expected 15-year battery life at typical settings, and can recharge in about 30 minutes at the default speed.
Why it matters for the market
- Expanding treatment options: An estimated 16 million U.S. adults live with urge urinary incontinence, a common symptom of overactive bladder. Many either do not respond to or discontinue medications due to side effects, creating demand for device-based therapies delivered in outpatient settings.
- Physician workflow: A short, minimally invasive procedure without sedation or imaging lowers barriers to adoption in urology and urogynecology practices and may broaden access beyond centers that routinely implant sacral neuromodulation systems.
- Competitive landscape: The approval underscores growing momentum around tibial nerve stimulation as a category complementing sacral neuromodulation and percutaneous tibial nerve stimulation. Several companies are pursuing implantable tibial systems; Medtronic positions Altaviva alongside its existing InterStim franchise. Claims that the approval makes Medtronic the only company with a full neuromodulation portfolio for bladder control are Medtronic’s characterization.
- Economic implications: If outpatient placement and limited maintenance hold in real-world use, health systems could see lower procedure and follow-up complexity versus more invasive alternatives. Payer coverage, coding, and pricing will be key to uptake.
Clinical and regulatory notes
- Altaviva’s approval follows the company’s pivotal TITAN 2 study. Medtronic did not release detailed outcomes in its announcement. Clinicians and payers will look for peer‑reviewed data on symptom reduction, durability, reintervention rates, and safety.
- The company did not disclose the FDA pathway or label specifics beyond treatment for urge urinary incontinence.
Go‑to‑market considerations
- Positioning: Altaviva gives Medtronic an implantable option along the tibial pathway to complement InterStim sacral neuromodulation, potentially widening its funnel of candidates who prefer less invasive therapy or who failed conservative care.
- Practice enablement: Training for office-based implantation, patient selection criteria relative to drugs, external tibial stimulation, botulinum toxin, and sacral neuromodulation, and post‑implant follow‑up protocols will shape rollout speed.
- Supply chain and service: Battery longevity, rapid recharge, and MRI compatibility are likely to be emphasized in value discussions with providers and payers; service models and warranty terms were not disclosed.
- Investor cadence: Medtronic plans an investor call Oct. 9 at 10 a.m. CST to discuss Altaviva and its market impact.
What to watch
- Reimbursement: Coverage policies, coding clarity, and payment rates for implantation and follow-up will determine adoption.
- Evidence: Publication of TITAN 2 outcomes, head‑to‑head or indirect comparisons versus drug therapy, botulinum toxin, external PTNS, and sacral neuromodulation.
- Competitive responses: Timelines for rival implantable tibial systems and any label expansions across the category.
- Utilization patterns: Uptake in office settings versus ambulatory surgery centers, and conversion rates from medications to device therapy.
- Safety and durability: Real-world data on adverse events, explant rates, and long-term battery performance.
Medtronic is headquartered in Galway, Ireland, and reported that its neuromodulation technologies have been used for bladder control conditions for more than three decades. The company did not provide pricing or launch timing for Altaviva in its announcement.