Consumers want a consistent experience and taste, and they expect their food and beverages to be safe. As the craft beer market expands, stringent federal compliance requirements demand that these startups implement food safety and quality programs. Compliance with federal regulations should be viewed as a method to protect the brand and provide guidance to improve systems, according to Eric Schaefer of Stone Technologies, a national systems integrator headquartered in Chesterfield, Missouri, that specializes in the food and beverage industry and is a certified member of the Control System Integrators Association (CSIA).
Preventive controls are risk-based, reasonably appropriate procedures, practices and processes employed to significantly minimize or prevent the hazards identified under the hazard analysis of the manufacturing process, says Schaefer. These procedures include sanitation procedures, employee training, a recall plan and Current Good Manufacturing Practices (cGMPs).
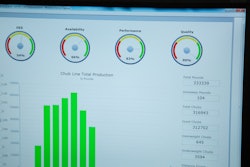
“Traceability and recordkeeping are a big part of our compliance activities,” states Brian Owens, head brewer for O’Fallon Brewery. “Documenting lot numbers, tracking yeast and other ingredients on the brew logs, as well as the packaging components, are critical to help us respond to an issue if needed. It is a lot of paperwork, but we are working to use less paper to make access to the documentation easier and quicker.”
Schaefer offers the following advice for craft brewers to keep their products safe:
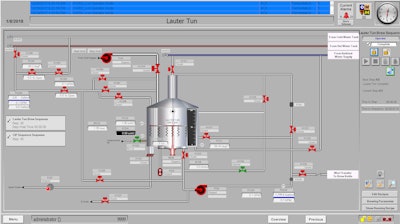
- Sanitary design with proper instrumentation and equipment: cGMPs cover sanitation and methods to properly clean equipment, but the equipment should be properly designed and cleanable. In addition, there must be adequate precautions to prevent cross-contamination with chemicals and other materials. Requirements for adequate cleaning should be measured with the correct instrumentation to ensure cleaning is performed properly.
- Standard operating procedures (SOPs): Craft brewers should document SOPs, including employee hygiene, food-handling techniques and cleaning processes. In addition to documentation developed by qualified personnel, other employees must be trained for awareness and daily practices. Automated procedures for production and cleaning assist in consistent operations and prevention of cross-contamination.
- Process/material documentation: The Food Safety Modernization Act (FSMA) and cGMP require records to be maintained for two years and available for inspection by FDA. Any instrumentation and controls for measuring and recording critical process parameters must be maintained and accurate for their purpose. FSMA also expands FDA access to records for foods that may cause serious health concerns as well as other foods that may have been similarly affected. While the Alcohol and Tobacco Tax and Trade Bureau (TTB) is not a part of FDA, FSMA gives TTB the ability to report incidents to FDA, where food safety issues are identified (TTB, 2015).
Educational materials are available through FDA, Brewers Association and Master Brewers Association of the Americas. Another resource is the Food Safety Preventive Controls Alliance.
To learn about how food and beverage processors are complying with complex FSMA documentation requirements, please read "Rising up to meet FSMA documentation challenges."
References
cGMP, F. (2015, September 17). Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food. Retrieved from FDA Regulations: www.regulations.gov/document?D=FDA-2011-N-0920-1979
FDA. (2014, April). Questions and Answers for Brewers/Distillers on the Original FSMA Proposed Rule for Preventive Controls for Animal Food. Retrieved from FDA.gov: www.fda.gov/food/guidanceregulation/fsma/ucm394991.htm
TB. (2015, July 31). TTB Newsletter | Weekly News. Retrieved from TTB.gov: www.ttb.gov/newsletters/archives/2015/ttb-newsletter073115.html