
Key Takeaways:
· Moderna wanted to eliminate the blind spots in the supply chain, such as shipment delays or weather impacts, by leveraging advanced thermal modeling.
· As more data is collected over time, the accuracy and reliability of predictive models improve, further enhancing the efficiency of logistics operations.
· Beyond reducing intercept rates and improving customer experience, Moderna has seen a reduction in waste in product and a reduction in carbon footprint.
The shipping of medicines and vaccines is a critical operation that must withstand high temperatures, shipping delays, and unforeseen issues so that the life sciences products can reach its destination safely. Today, that means leveraging technology to better predict a packaging fail, getting ahead of extreme weather, and ensuring a timely delivery.
Earlier this year at TempPack, part of the ISTA Forum, Jeff Lander, Director, Global Logistics Engineering for Moderna and Paul DellaVilla, Director of Digital Solutions and Services, Cold Chain Technologies (CCT), gave a presentation titled, Leveraging Real-Time Tracking, Location Data, and Real-Time Thermal Modeling to Reduce Shipment Risk. They shared the integration of real-time tracking and thermal modeling to enhance pharmaceutical logistics, focusing on proactive risk management and customer experience.
I sat down with them both after the event to dig deeper into these technologies and learn about the tangible benefits that the solution offers.
HCP: Jeff, tell us a bit about the solution; What prompted Moderna to adopt real-time tracking and thermal modeling in its logistics?
Lander: The solution was inspired by the “Amazon Prime experience” where I know where my box is, I know when it's going to be delivered, and I know how long it's going to last. We wanted to eliminate the blind spots in the supply chain, such as shipment delays or weather impacts, by leveraging advanced thermal modeling. This approach allows us to provide a customer-centric experience where both Moderna and its customers can track shipments in real-time and proactively address any potential issues.
As an industry, we do a good job at doing some preventative guidance in terms of modeling different scenarios before a shipment leaves or taking a large data set and doing some retroactive analysis of that larger data set. But the big blind spot in the industry is what happens in between… a shipment leaves, something gets delayed, misses a flight, weather impact, etc. We have that blind spot of what do we do? Do we intercept it? Do we let it ride? How long will my box last if it gets delayed? And as a result, we were able to leverage advanced thermal modeling by CCT to have better insights into our supply chain.
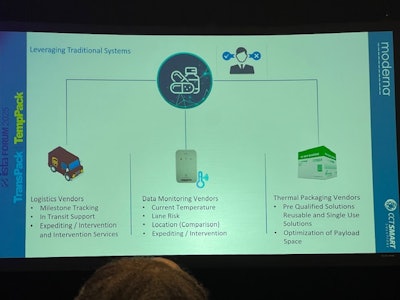
Lander: Each stakeholder in the supply chain receives the appropriate amount of information. We have access to comprehensive data to make informed decisions, while customers receive essential updates without being overwhelmed.
DellaVilla: We wanted to make sure that each part of that group had the right amount of information. The Moderna team has a wider array of information to allow them to make better decisions and get the right information down to their customer. A pharmacist doesn't want to know every single piece of detail of what's coming, but they need the right amount of information to decide what to do with the boxes when they get there.
HCP: Can you elaborate on the proactive versus reactive approach to shipment risk?
Lander: The feedback that we had from our customers was, ‘how do we know when it's going to arrive’ and ‘how do I know my product is OK?’ So that's where we had that customer-centric approach to introduce this real-time active monitoring. Before implementing this technology, we relied on chemical or electronic indicators with a wide tolerance for error. Now, with real-time monitoring, we can assess data dynamically and as a result, we reduced our intercept rate from 1.9% to 0.3%. So, a significant reduction in a number of shipments that needed to be intercepted and that would have been pulled out of the field because we were using strictly a qualified duration in a laboratory as opposed to using the dynamic thermal modeling from CCT, which ingests the thermal model or thermal performance of the box in combination with the ambient weather data to spit out and calculate a predictive time that that box will last.
So, we were using that dynamic model to be able to say, ‘OK, well, our box in a lab is qualified for 36 hours. However, we have a frozen product, it's the middle of November. That box realistically will last 2,3, even sometimes 4 days longer than the worst-case testing, which is like 95 degrees in the middle of July, or 100 degrees in, you know, in California.’ This proactive approach allows us to optimize shipping lanes and reduce carbon footprint by shifting from air to ground transportation where feasible.
HCP: What advice would you give to companies looking to adopt similar technologies?
DellaVilla: The first step is a willingness to change how things are done. We’re a slow industry to change. Many companies are hesitant due to a lack of understanding or fear of impacting patient safety. So, you know, a forward-thinking customer like the team at Moderna, they really gave us the ability to try to integrate some of these newer technologies. As Jeff mentioned, most people are using qualified thermal packaging. What can be done after that though, really requires companies to start to think outside the box with integrating things like real-time modeling where we can identify in real time if or when our box is going to fail.
I've played in both sides of the world, both, you know, working with pharmacy and their equipment on site, as well as the distribution and the one thing I've figured out across the board is that pharmacists are focused on getting medicines to patients. They don't want arduous things that kind of take away from their day to day. So, you know, these types of technologies can really start to add value to their day by taking away a lot of the concern. They're not looking at a data logger, they're not looking or trying to decipher a chemical indicator. They're getting an email. Your box arrived, it's here, it's ready to use. Here are the instructions for what to do with it. It's a very clean process from their end.
Lander: I've spoken to a lot of different companies about this, and a lot of people have asked me questions on how we approached this and how we got started. I will say that the two common themes that I see from companies hesitant to start is that they are not looking at the total cost of ownership, which is an absolute critical and pivotal part of this. The other piece of it is a lot of companies don't know where to get started because they try and boil the ocean too fast. Identify your biggest pain one or two pain points and start small, get comfortable with it, and expand from there.
HCP: Have you seen positive customer feedback since implementing these changes?
Lander: We went into this strictly looking at an enhanced customer experience; we weren't expecting to reduce intercept rate. We weren't expecting to be able to have, you know, shipment level optimization, looking at next day air to second day air to ground point. We knew that those benefits would come inherently. But we have seen a significant reduction in call volume to our call center, over 70%, indicating fewer customer complaints and inquiries. The simplicity of the device, requiring no customer engagement, has been a major factor in improving the customer experience.
HCP: What are the other tangible benefits you've observed?
Lander: Beyond reducing intercept rates and improving customer experience, we have seen a huge reduction in waste in product and a reduction in our carbon footprint. Each intercepted shipment previously required a replenishment process, which we have now minimized.
HCP: How does the technology work, and how can it be integrated into existing systems?
DellaVilla: I would say we utilized one or two pieces of novel technology, but the majority of it was the right combination of the technology at the same point.
You know, with Domino’s you can track your pizza from the oven all the way through to shipping; that technology exists in the market. Plus, we all know when our Amazon packages are showing up. So, it's taking that technology and then pairing it with the overlay of that real-time tracking; finding the right fit of getting the right data through and the right cost level as a part of it.
What we brought to the table, which is I do think that's something new in the market, is the ability to parlay the logistics information. The tracking information we get from the data logger and then information from weather that we're getting from different sources in there to predict when the box is going to fail. So, each of those pieces offer, you know, a very unique set of values, but when you pair them all together, the sum of the whole is far more than the sum of the parts in that piece.
HCP: What role does data play in enhancing these logistics solutions?
Lander: Data is at the core of these logistics solutions, enabling companies to make informed decisions and optimize their operations. This data-driven approach allows for continuous improvement, as companies can identify trends, predict potential issues, and implement strategies to mitigate risks. As more data is collected over time, the accuracy and reliability of predictive models improve, further enhancing the efficiency of logistics operations.
HCP: There seems to be a boom in refrigerated biologics and pharmaceuticals and some of the more popular auto-injector style drugs. Have you seen an uptick in cold chain and refrigeration requirements and did that have any weight in your decision to use this kind of technology?
Lander: You know cold chain medicines, especially, have been on significantly on the rise. And there's a lot of research papers even the last year or two years that have predicted the growth of cold chain medicines, substantially, now, but also into the future. And I see a huge opportunity, especially going direct to patient. I can tell you right now that's definitely an underserved or under-watched area going D2P, especially with the boom of the GLP-1s; you're talking about a huge number of, of volume of shipments. I can see this type of technology being utilized there.
HCP: Can smaller companies also benefit from this technology?
DellaVilla: Absolutely. While larger companies like Moderna have specific needs, smaller companies can also adapt these technologies to their supply chains. It's about finding the right combination of technology that fits their profile and risk tolerance.
HCP: What is the future of this technology in pharmaceutical logistics?
Lander: There’s a big opportunity here—not by using internal data loggers, but by collecting enough shipment data to build and continually improve a thermal model using machine learning. With enough data, you could achieve high accuracy (within 5%), allowing you to potentially eliminate the need for internal temperature monitors by relying on the model's predictions.
Looking ahead, I see the use of external ambient sensors or smart shipping labels that collect environmental data. Since current API weather data lacks shipment-level granularity, these labels could fill the gap. As the model improves over time, a low-cost external label (a few cents or a dollar) could replace expensive internal monitors, making the system more scalable and robust.
In short, a low-cost, external sensor could improve model accuracy enough to eliminate the need for more complex or less reliable internal devices, while also simplifying the user experience.
DellaVilla: It's really finding the right way to cobble together these different pieces of technology for your specific supply chain. GLP-1 is going to be different than a COVID vaccine, which is going to be different than an oncological product.
From our end, it's people who are willing to take the chance at trying to look and pull together new technology, and then, look at different ways of trialing it without adding risk to their supply chain. So you know, there's a lot of good learnings and things that came out of this project that we're trying to kind of evangelize a little bit in the space in terms of technology adoption, ours or other people, you know, this is, this isn't just a Cold Chain Technologies item; this is the type of thing that can impact the industry as a whole.
Looking to get started? Jeff Lander’s final piece of advice for companies looking to get started on a program like this one is to start small.
“It comes down to starting small and not trying to boil the ocean, because once you start diving into all the details, you end up scaring yourself from getting started. And since you don't know where to get started, you don’t start at all.”






















