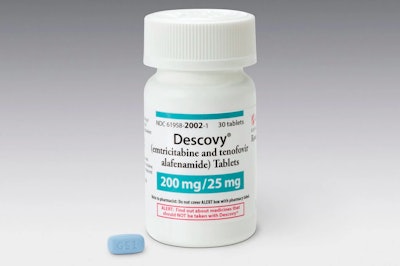
The FDA just approved a new drug for the prevention of infection with HIV, but it comes with a caveat. According to a recent New York Times article, Gilead’s Descovy was only tested on men and transgender women and is therefore only approved for those groups. The approval explicitly excludes cisgender women (women who identify with their female birth sex), and didn’t outline a plan for making the drug available to them. Critics claim the exclusive approval sets a dangerous precedent, as companies are able to avoid the expensive trials necessary to test drugs in cisgender women.
The first FDA-approved drug for HIV, Truvada, is also made by Gilead. Both Truvada and Descovy are daily pills intended for pre-exposure prophylaxis (PrEP). Truvada’s patents expire next year, when less expensive generic versions are expected to hit the market. The FDA is requiring Gilead to test the drug on cisgender women, and the company plans to do so with 1,500 high-risk women in southern Africa by the end of 2020.